Body Armor Grown in a Lab? Why Not, Say Synthetic Biologists
Can a tiny living cell be converted into a mighty manufacturing facility? It already has.
What may seem like a futuristic fantasy is already an everyday reality for Northwestern scientists like Michael Jewett. He and his colleagues at the Center for Synthetic Biology (CSB) are using tools and concepts from physics, engineering, and computer science to create biological systems that can perform novel, specialized functions with potential for applications in fields ranging from energy to biomedicine. Recent efforts in the Jewett lab break new ground and also hold promise for national defense and security. His work also has potential for helping accelerate drug development and ramping-up inexpensive quick-test tools to fight the COVID-19 pandemic.
 Professor Michael Jewett is a chemical engineer and among the Northwestern faculty who are advancing the field of synthetic biology.
Professor Michael Jewett is a chemical engineer and among the Northwestern faculty who are advancing the field of synthetic biology.
Found in all living cells, ribosomes are complex machines that make the proteins needed for many cell functions, such as repairing damage. They do that by building chains of amino acids based on the instructions given to them by an organism’s genetic code, as embedded in DNA. CSB researchers are among those exploring how to harness the power of ribosomes and “reprogram” them for new purposes, removing them from the cell structure the way one might remove an engine from a car. They then rev up this engine to get it to produce macromolecules called polymers that can be used to create a wide variety of substances: everything from protective gear for military applications to a new class of antimicrobial drugs that fight antibiotic-resistant bacteria.
“We’re focusing on repurposing the ribosome of the cell to make new kinds of polymers that address societal and, in this case, also Department of Defense needs,” says Jewett, the Charles Deering McCormick Professor of Teaching Excellence and the Walter P. Murphy Professor of Chemical and Biological engineering. He also directs the CSB, which has received a grant from the U.S. Army to further this research, strands of which are also underway at three other universities. While synthetic chemistry also performs some of the same functions, synbio researchers believe the biological polymers will prove more precise and durable over time.
On April 14, Prof. Jewett appeared on WGN News to explain how his work can help fight COVID-19.
“Materials are often made of polymers,” Jewett notes. “Could you make, for example, next-generation Kevlar or new kinds of nylons that have better properties, or could be synthesized in a more sustainable way, because you have a biological system making it?”
Published in Nature Communications, the recent research of Jewett and his colleagues has involved developing a set of design rules to establish how ribosomes can incorporate new types of monomers, which form polymers when bonded with identical molecules. The research has shown that the ribosome can accommodate more types of monomers than initially anticipated, and by creating the design rules, researchers no longer need to rely on time-intensive trial and error.
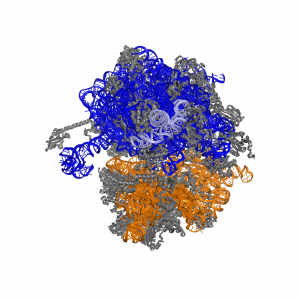 The ribosome is the "chef" of translation. Researchers at Northwestern's Center for Synthetic Biology are changing the catalytic ribosomal RNA (shown here in colors) to enable the synthesis of next-generation materials and new medicines.
The ribosome is the "chef" of translation. Researchers at Northwestern's Center for Synthetic Biology are changing the catalytic ribosomal RNA (shown here in colors) to enable the synthesis of next-generation materials and new medicines.
This work sits at the intersection of synthetic biology and materials science, and the Army has an interest in two aspects: whether the research could lead to synthesizing new kinds of materials for use in everything from stronger soldier protection to better tents, and whether these investigations could lead to the ability to make products in austere conditions, on site and on demand, Jewett says.
“Instead of using large, centralized facilities to make products that you would then ship, could you have a plastic bag with a freeze-dried powder that, when you add water, makes the material?” he says. “It’s kind of like what 3-D printing is doing. This process is completely changing the game of how we make plastics and other materials.”
Such materials are difficult to prepare using chemical synthesis methods, but synthetic biologists like Jewett believe the use of engineered ribosome could lead to manufacturing of multiple new classes of sequence-defined polymer families. In turn, these developments could lead to a wealth of possibilities: advanced personal protective gear, lightweight materials, sophisticated electronics, fuel cells, and nanofabrication. “These are all topics key to the protection and performance of soldiers,” Jewett says. “Our approach may even harness one of the most salient features of biology — the ability to evolve, in search of new classes of materials.”
Jewett sees this research as merging the best of chemistry and biology. “This multidisciplinary combination means being able to control those molecular ensembles that do all of the genetic code reading, controlling and finely tuning a process that’s developed over 3.5 billion years, but now using it to make some new product,” he says. “One of the things that’s so exciting about this recent paper we published is we significantly expanded the scope of possible monomers that could be used to make polymers above what had existed.”
New ‘Chefs’ Make New ‘Food’
Scientists already have harnessed the power of ribosomes to make products like insulin for diabetic patients, antibodies against cancer, and enzymes that go into laundry detergent to help dissolve ketchup stains, Jewett says. But the proteins in those cases are all made of the same type of amino acid — which he likens to being the same cuisine, made by the same chefs.
“What this project is about, is making new chefs,” he says. “How do I make new ribosomes that make new types of cuisines? At a high level, this project is interesting in asking whether we can teach the ribosome to make new classes of polymers that could address societal needs in new areas like therapeutics, sustainable materials, or bioelectronics. That’s a really exciting step forward for us.”
An extra hurdle comes from the fact that cells need ribosomes to live, and so using living cells for this work would mean fighting a tug-of-war between evolutionary imperatives and the engineering objectives of researchers. Addressing that challenge is the “cell-free” innovation, a process in which the ribosomes are removed from cells. Doing so, says Jewett, then “frees up flexibility to move beyond monomers that biology has evolved to use over billions of years to use new kinds of monomers that can be polymerized by these new ‘chefs.’” Jewett’s new research developing a platform for engineering ribosomes in a cell-free context shows this is possible.
More broadly, Jewett sees the Center for Synthetic Biology poised on the vanguard of a new bio-economy that will explode from this foundational research, much as the computer industry emerged out of electrical engineering. “We are now entering this era of being able to control biological systems to benefit society,” he says. “We’ve got this outstanding multidisciplinary research center that attracts faculty talent from across the medical school, the engineering school, and the college of arts and science at Northwestern.”
Being able to expand a cell’s genetic code to synthesize new classes of polymers has been a tantalizing dream, Jewett says. “It’s actually emerged as one of the most exciting opportunities at the interface of chemical and synthetic biology, for at least the next decade,” he says. “We’re just at a point where we have had this wave of fundamental understanding that allows us to manipulate and control biological systems, and now we’re able to transition that capacity into products that will fundamentally transform our economy.”
Doing so will require enrolling ribosomes in “cooking class,” where they learn to make these other cuisines, Jewett says. But the science is evolving fast. “Three years ago, we couldn’t even ask the questions we’re asking today,” he says. “What’s fun is we’re hitting it in stride and we foresee tremendous growth.”