Understanding and Combating Virulent Viruses
The spread of the coronavirus from China is raising fears of a global pandemic. At Northwestern, multidisciplinary scientists are increasing our knowledge about various viruses and how they mutate and function. Northwestern Medicine physician Gregory Smith recently shared his insights about how his lab is working to unlock the secrets of viral transmission and help safeguard health.
 Greg Smith, Microbiology-Immunology
Greg Smith, Microbiology-Immunology
What are your research interests?
An ancient group of viruses that has co-evolved with the animal kingdom: the herpesviruses. These agents are more related to viruses of bacteria (bacteriophage) than they are to other animal viruses, and they are remarkably successful. Individual viruses from this group infect mammals ranging from mice to elephants to seals, as well as ourselves. We study one such virus that is carried by the majority of people on earth: herpes simplex virus (HSV), and a related but particularly virulent veterinary virus that infects many farm and companion animals called pseudorabies virus (PRV). Of particular interest for me is the propensity of both of these viruses to invade the nervous system.
What is the ultimate goal of your research?
We are striving to obtain a working understanding of these viruses, which I like to think of as nano-machines, and to translate this knowledge into tools to develop vaccines and advanced viral vectors for a range of applications, including oncolytics to treat cancer and neural tracers to map brain circuitry. In parallel to this, we are studying why in rare circumstances these viruses cause severe encephalitic infections.
How did you become interested in this area of research?
Since my graduate thesis research, I have been interested in what pathogens can teach
us about ourselves. I am particularly fascinated by the molecular mechanisms employed
by bacteria and viruses to manipulate the biology of our cells and how they manifest in disease. In graduate school, I was researching a food-borne bacterial pathogen, Listeria monocytogenes, which manipulates the actin cytoskeleton to rocket between cells while hiding from the immune system. As amazing as Listeria is, it causes only sporadic outbreaks that generally involve people with compromised immune systems. By contrast, HSV has spread to the majority of the human population: essentially the biggest outbreak possible. And it infects us for life by hiding in our nervous system. How could I not become interested?
What types of collaborations are you engaged in across campus (and beyond)?
The people in my lab are engaged in highly interdisciplinary research. Our bread and butter is molecular virology with healthy doses of cell and neuronal biology. We’ve pioneered genetic methods to manipulate the virus and live-cell imaging techniques to watch individual viruses “running” down axons to establish infections in sensory ganglia. All of this is augmented by our collaborations. On the cell biology side, Volodya Gelfand, PhD, the Leslie B. Arey Professor of Cell, Molecular, and Anatomical Sciences in the Department of Cell & Developmental Biology, is an exceptional scientist and colleague who has made some of our recent discoveries possible. Drs. Gary Pickard and Patricia Sollars (University of Nebraska at Lincoln) are long-term neuroscientist collaborators who are core to our research program. In fact, together with Dr. Pickard and Dr. Katya Heldwein of Tufts Medical School, we have founded a company, Thyreos LLC, to advance some of the vaccine technology that has resulted from our research. My lab also participates in a scientific consortium with Drs. Jean-Laurent Casanova and Shen-Ying Zhang (Rockefeller University), Dr. Luigi Notarangelo (National Institutes of Health) and Dr. Lorenz Studer (Memorial Sloan Kettering Cancer Center) to unravel the genetic predispositions that result in lethal HSV encephalitis.
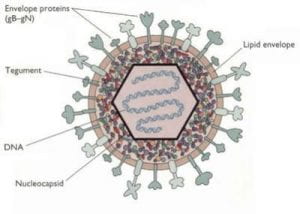 Structure of the herpes simplex virus
Structure of the herpes simplex virus
How is your research funded?
We are currently funded entirely by the National Institutes of Health (NIH) with prior support coming from the Burroughs Wellcome Fund, Schweppe Foundation, Cold Sore Research Foundation and Life Sciences Research Foundation. The NIH funds projects to study the neuroinvasive properties of these viruses as well as the genetic in-borne errors that predispose to encephalitic infections.
Who inspires you?
My number one inspiration is my postdoctoral mentor, Dr. Lynn Enquist (Princeton University). His love of research and discovery combined with his supportive and well-meaning nature is a rare combination among the most successful in this field. Dr. Julie Theriot (Stanford University) was an early role model both because of her brilliance and passion for science.
This story originally appeared in the Feinberg School of Medicine’s Breakthroughs, December 2019.